PPM schedule impact on the reliability and the performance of the device
Scheduled PPM is a series of actions done to avoid disruptions that cause the business to suffer significant losses. Understanding and being aware of what needs to be done by early realisation of the design state of machinery and equipment is known as preventive maintenance. After that, the necessary steps are done to perform the services, which include cleaning, lubrication, and spare component replacement, and the periodic checks are initiated. As a result, there is a decreased likelihood of machine downtime and an increase in machine reliability. Understanding the machine’s failure rate through analysis and study of failure times is essential to the preventive maintenance process. The machine is in the useful life stage if the failure times exhibit an exponential distribution. In this instance, the equipment requires corrective maintenance rather than preventive maintenance, which will lessen the likelihood of a problem. The machine must, however, adopt a scientific approach to preventive maintenance if the failure times are longer and follow different distributions, such as a normal distribution, Weibull distribution, or other probability distributions. In this scenario, it is very helpful and minimises the likelihood of unexpected failure while also minimising downtime. Additionally, it causes the machines’ performance to become more efficient.
Technology may introduce new characteristics, materials, or manufacturing methods that can improve medical devices. Sensors, data processing, and connectivity can improve diagnostic precision and patient monitoring. Medical equipment enhances care. Equipment failure and unavailability in healthcare institutions hinder public care. Poor medical equipment management and maintenance by the responsible party causes problems. Bio engineering thrives. New technologies improve disease and deficiency treatment. IMDs improve computing, decision-making, and communication. IMDs have security and privacy vulnerabilities that could compromise the implant and the patient’s health, according to several computer security studies. Monitoring medical equipment’s lifespan improves availability, performance, and safety. Medical equipment condition evaluation input parameters were extracted and categorised in this systematic review. Strategies improve medical device companies’ organisational capabilities for growth and competitiveness. Risk management (RM) is essential to the functional safety and regulatory approval of modern medical devices.
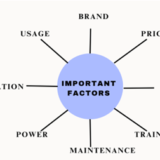
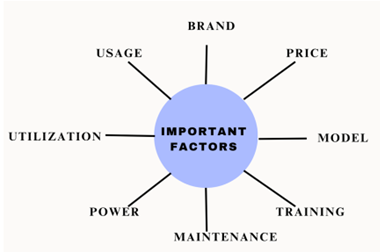
Important factors that need to be considered while purchasing and maintenance:
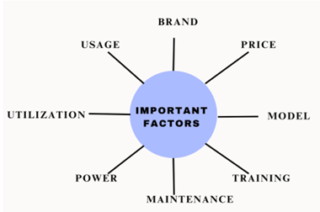
Brand- Choosing the right brand which delivers high efficiency.
Price- Negotiating the prices and services while purchasing high volumes of equipment.
Model- Preferring the right model and same model within the facility.
Training- Good training to the healthcare providers which reflects in right usage of equipment.
Maintenance- Maintaining the equipment depends on the department and usage should be considered.
Power- In few cases good power source can avoid the early breakdown.
Utilization- Utilizing the maintenance services, discounts and warranties provided by the companies.
Usage- Depending on the usage of the equipment, maintenance should be carefully monitored.